- Analyzers
- Optics & Sources
- Technologies
- Support
- About
A Comparison of Chlorine Levels in Aromatics Using Monochromatic Wavelength Dispersive X-ray Fluorescence and Microcoulometry
BACKGROUND
Petroleum refining processes have evolved over the years to maximize efficiency and output as crude oils are turned into finished products. One such evolution has been the increased level of quality testing done on petrochemicals such as aromatics. This shift in attention on quality and rigor makes sense, as the International Energy Agency reported in 2018 that “Petrochemicals are set to account for more than a third of the growth in world oil demand to 2030, and nearly half the growth to 2050, adding nearly 7 million barrels of oil a day by then. They are also poised to consume an additional 56 billion cubic metres (bcm) of natural gas by 2030, and 83 bcm by 2050” 1 .
From a capacity standpoint, refineries have already begun to respond. According to the Hydrocarbon Processing 2019 Industry Outlook, petrochemical capacity expansion makes up most CAPEX projects in the refinery space at 37%. By the reported numbers, this is an expected 474 projects out of 1,312 total projects, which accounts for roughly $560B in total CAPEX spend across the globe 2 . With this in mind, refineries must continue to make difficult decisions surrounding testing methods for their petrochemical applications, such as aromatics, as these projects move through the planning phase and into the execution phase.
CHALLENGE
Today, petroleum professionals use analytical equipment to monitor for chlorine in their aromatics, which can include xylene and benzene. Aromatics and finished products testing may be included in the product specification. These tests are typically done as a quality control check. A low-level sub-ppm performance is critical in the measurement of aromatics, as most aromatics come in the form of organic chlorine, which is typically present in very low concentrations.
Fortunately, there is more than one test method to accommodate this measurement criterium; however, parameters such as test time, sample preparation, and precision can vary widely depending on the test method used. These parameters are all critical components for petroleum professionals trying to balance a wide set of analytical needs for their lab. With such parameters in place, it isn’t always simple to determine the most appropriate test method that meets a lab’s specific needs.
Two common ASTM standard test methods for chlorine are D7536, Chlorine in Aromatics by Monochromatic Wavelength Dispersive X-ray Fluorescence Spectrometry (MWDXRF), and D5808, Organic Chloride in Aromatic Hydrocarbons and Related Chemicals by Microcoulometry. This paper will break down the differences between each of these test methods, as well as review data from the ASTM Aromatic Hydrocarbons Proficiency Testing Program (PTP).
Use of D5808 requires a liquid sample to be injected into a combustion tube for analysis. This combustion tube is maintained at a temperature of 900°C and has a flowing stream of oxygen and argon carrier gas. According to section 4.1 of the test method: “Oxidative pyrolysis converts the organic halides to hydrogen halides that then flow into a titration cell where it reacts with silver ions present in the electrolyte” 3. The silver ions are then coulometrically replaced, and this electrical work of replacing the silver ions is the measure of the organic halides in the sample.
Microcoulometry involves the use of a furnace, tubing, and syringe injection of sample for measurement. Part of this process also involves stirring, which is done magnetically by the titration cell – this parameter must be closely overseen to ensure that stirring speed does not exceed a threshold that will cause damage to the electrodes. Additionally, microcoulometry involves the consumption of gasses for sample preparation.
Monochromatic Wavelength Dispersive X-ray Fluorescence (MWDXRF) – D7536 4Alternatively, use of D7536 requires a sample to be pipetted into an X-ray sample cup. The cup is sealed with sample film, vented, and placed into the analyzer for analysis. Users enter measurement parameters which include measurement time, repeats, and selecting a calibration.
NOTE I: For general MWDXRF analysis, XOS has published a recorded webinar on best practices for sample preparation. This webinar was published using a sulfur analyzer, but many of the tips and recommendations apply when analyzing for chlorine content as well. xos.com/SindieBestPractices
MWDXRF works using high-intensity X-rays that excite the elements of interest within a sample. Upon exposure, fluorescent X-rays are emitted from the sample at energy levels that are unique to each element. To isolate the chlorine signal and reduce noise, traditional WDXRF utilizes a filter and a collection crystal before the chlorine signal reaches the detector. With MWDXRF, however, an additional excitation optic is used to monochromate the sample which improves noise reduction, ultimately leading to better precision. See the Technology Brief segment at the end of this paper to learn more.
ASTM PROFICIENCY TESTING PROGRAM
ASTM conducts an aromatic hydrocarbon Proficiency Testing Program (PTP) twice a year. In each PTP session, ASTM sends aromatic hydrocarbon products or feedstocks to various participant sites for analysis of multiple sample properties. Each participating laboratory performs analysis following ASTM methods for these test parameters. The ASTM PTP chlorine results using the previously-discussed MWDXRF and microcoulometry methods can be found can be found in the Study Results section.
Study Results
The data used in this paper was gathered over the course of four testing sessions between the Spring of 2016 and Fall of 2018. These samples were either unknown or were doped with chlorine compounds, unbeknownst to the PTP participants. The aromatic hydrocarbon program dopes some of the samples so that there are detectible chlorides for the measurements.
As we can see in Table 1, MWDXRF method D7536 demonstrates closer or equivalent accuracy to a doped nominal value against microcoulometry method D5808 100% of the time. In one case, D7536 was an exact match with the doped value, and two other scenarios provided results with less than a 0.1 ppm difference from the value.
| Table 1 - ASTM PTP Data | |||
|---|---|---|---|
| D5808 (mg/kg) | D7536 (mg/kg) | Doped Value (mg/kg) | |
| Fall 2018 | 0.35 (± 0.20) | 0.46 (± 0.17) | 0.5 |
| Spring 2018 | 0.9 (± 0.30) | 0.9 (± 0.30) | 1 |
| Spring 2017 | 0.5 (± 0.30) | 0.8 (± 0.20) | 0.7 |
| Fall 2016 | 0.3 (± 0.20) | 0.5 (± 0.20) | 0.5 |
We can see a trend more clearly when we look at this same data graphically. The orange line, representing MWDXRF method D7536, more closely and consistently aligns with the gray line, which represents the doped value. The blue line, representing microcoulometry method D5808, demonstrates a high degree of accuracy as well, but does not align as closely with our gray line value as the MWDXRF method.
Figure 1:ASTM PTP Data
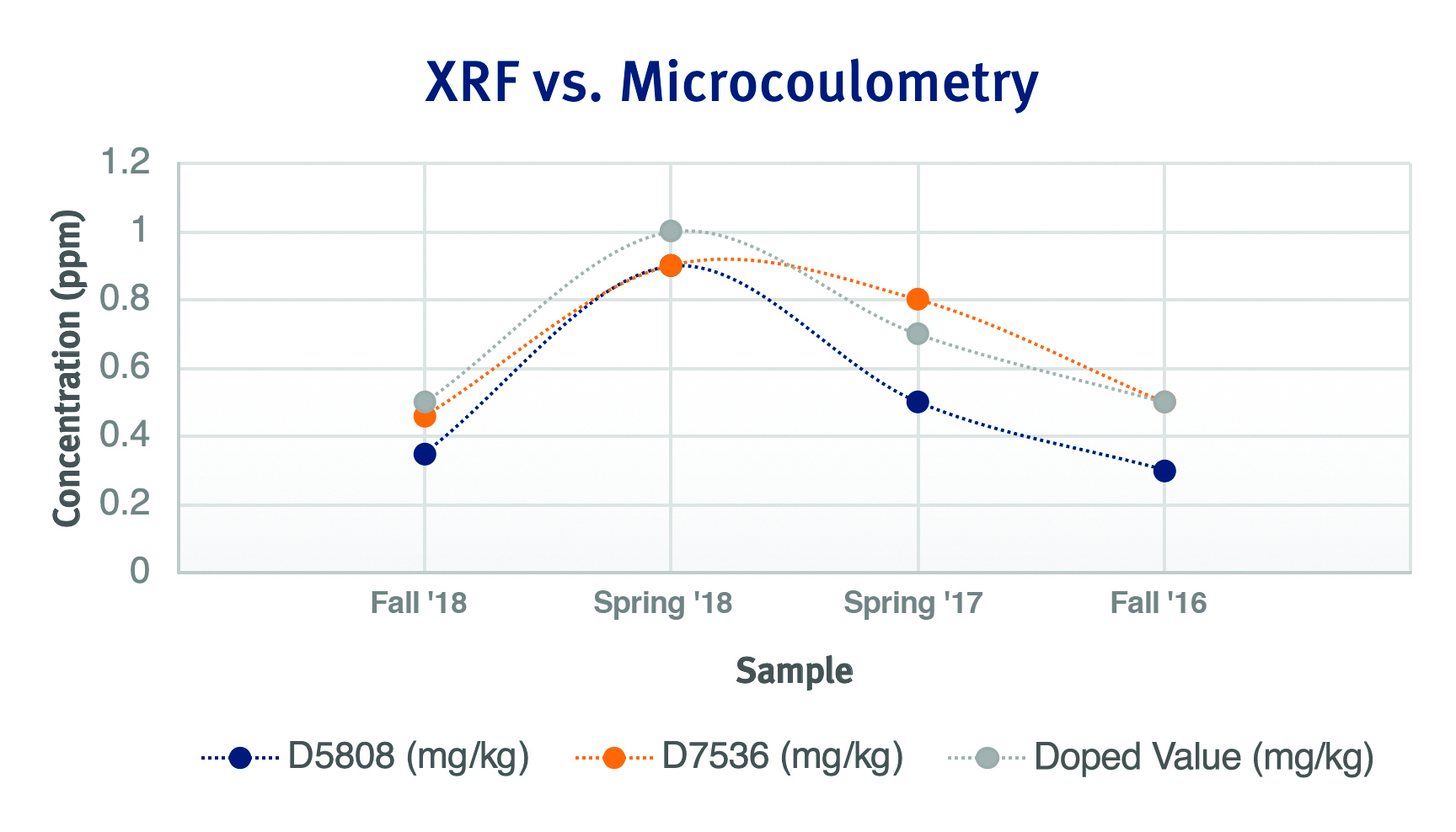

This data, obtained using XOS Clora analyzers, demonstrates that MWDXRF method D7536 provides petroleum lab professionals with a high degree of measurement accuracy, ensuring that the number reported by the analyzer is the correct number.
What about precision? Section 16 of D5808 and D7536 lists the precision criteria for each method. Tables 2 and 3 below, list the calculated precision values from each method. As seen from the method tables below, the method repeatability and reproducibility for D7536 is better than D5808 within the range of interest. As a quick reminder:
- REPEATABILITY (r):The difference between two results run by the same operator on the same analyzer for the same sample.
- REPRODUCIBILITY (R):The difference between two single and independent results obtained by different operators in difference laboratories using different analyzers on the same sample.
| Table 2 – ASTM Method D5808 Precision Values | ||
|---|---|---|
| Chloride Concentration (mg/kg) | Repeatability (mg/kg) | Reproducibility (mg/kg) |
| 0.7 | 0.7 | 1.3 |
| 1 | 0.7 | 1.3 |
| 2 | 0.7 | 1.3 |
| 5 | 0.7 | 1.3 |
| 7 | 0.7 | 1.3 |
| 10 | 0.7 | 1.3 |
| Table 3 – ASTM Method D7536 Precision Values | ||
|---|---|---|
| Chlorine Concentration (mg/kg) | Repeatability (mg/kg) | Reproducibility (mg/kg) |
| 0.66 | 0.26 | 0.41 |
| 0.75 | 0.28 | 0.43 |
| 1.00 | 0.30 | 0.50 |
| 2.00 | 0.39 | 0.71 |
| 5.00 | 0.49 | 1.12 |
| 7.00 | 0.54 | 1.32 |
| 10.07 | 0.60 | 1.59 |
Looking back at our data in Table 1, we will pay closer attention to the numbers within the parentheses for both D7536 and D5808. Seeing the uncertainty ranges for each respective data set, we can see that the results for each fall within their respective test method reproducibility. Looking at two of the four sets of data, the uncertainty ranges for D7536 are tighter than those of D5808, which have a much wider range of error. The exception to this is seen for the Spring 2018 and Fall 2016 sets of data, wherein the results for both methods are identical. From here we can see that from both a precision and accuracy standpoint, XRF fares better than microcoulometry.
NOTE II: It is worth mentioning that specifications for xylene and other aromatics reference chloride and not total chlorine. Chloride refers to the outer or bonding electrons in the chlorine atom. Clora analyzers, as utilized in the PTP study, detect the presence of chlorine atoms by looking at the inner shell electrons, meaning that Clora analyzers measure total chlorine. Typically, chlorine present in the samples of interest at a refinery site are present in the chloride form. Therefore, if the only chlorine content present in the sample is in the form of chlorides, and Clora measures the total chlorine content present in the sample, then Clora, by extension, is able to report the results of chloride content in the sample of interest.
CONCLUSION
When measuring aromatics, precision and accuracy at low concentrations are critical. From the ASTM data shown, MWDXRF analyzers, particularly Clora,demonstrate excellent accuracy and precision when measuring samples against microcoulometry. The ability to measure total chlorines with minimal sample preparation and high performance, with results ready within minutes, is invaluable to refiners looking to protect their bottom line – and for these specific needs, Clora delivers on all fronts.

Clora® is a compact analyzer to measure total chlorine in liquid hydrocarbons such as aromatics, distillates, heavy fuels, crude oils, and aqueous solutions. Clora delivers unprecedented accuracy and precision for petroleum and petrochemical applications where ease-of-use, reliability and measurement speed are critical.
Technology Brief: Monochromatic Wavelength Dispersive X-ray Fluorescence (MWDXRF)
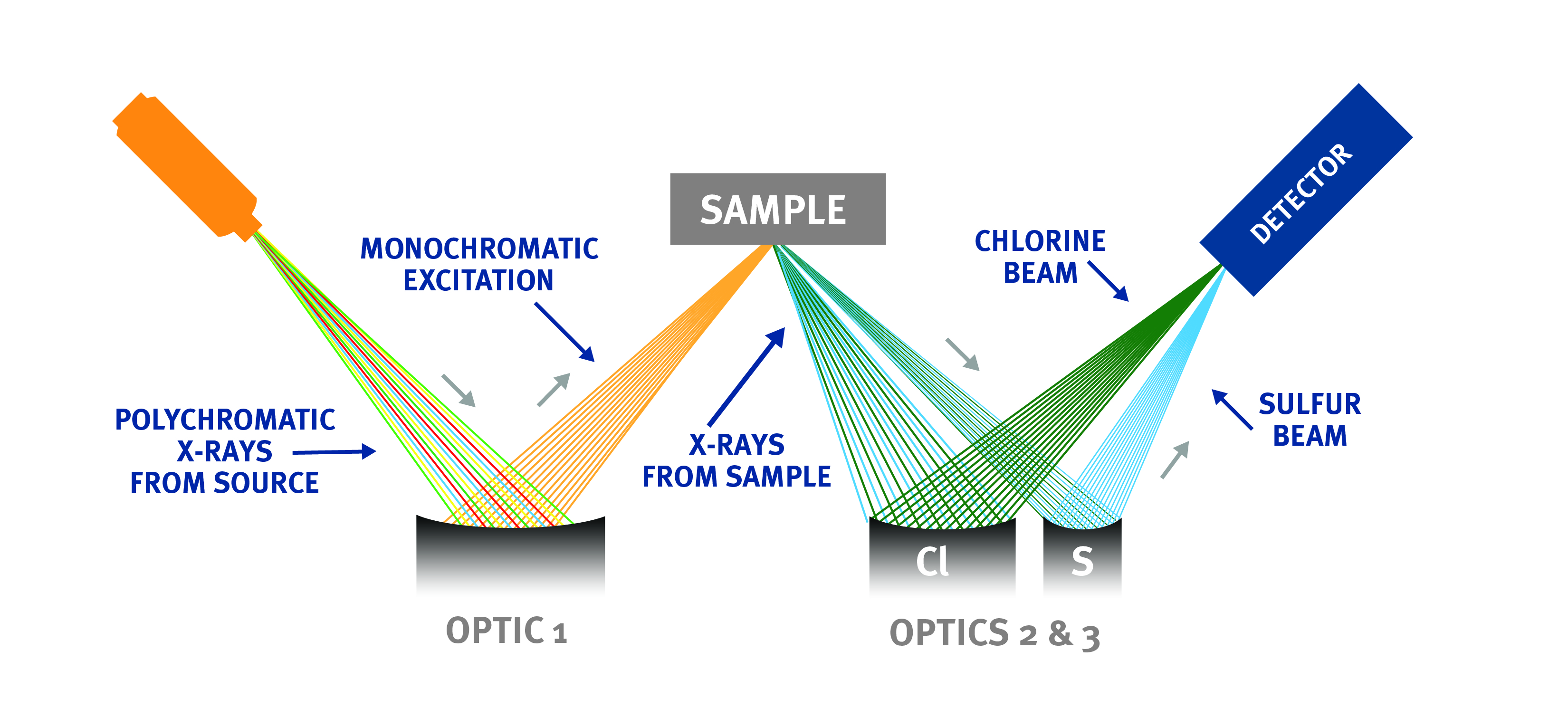
Monochromatic Wavelength Dispersive X-Ray Fluorescence (MWDXRF) utilizes state-of-the-art focusing and monochromating optics to increase excitation intensity and dramatically improve signal-to-background ratio compared to traditional WDXRF instruments. This enables signifi cantly improved detection limits, precision, and a reduced sensitivity to matrix eff ects. A monochromatic and focused primary beam excites the sample, and secondary characteristic fluorescent X-rays are emitted from the sample. A second monochromating optic selects the chlorine characteristic X-rays and directs these X-rays to the detector. MWDXRF is a direct measurement technique and does not require consumable gasses or sample conversion, delivering robust and low-maintenance analyzers with dramatically lower detection limits and faster response times.
REFERENCES
- https://www.iea.org/news/petrochemicals-set-to-be-the-largest-driver-of-world-oil-demand-latest-iea-analysis-finds
- https://www.hydrocarbonprocessing.com/resources/webcasts
- ASTM D5808 – 18, Standard Test Method for Determining Chloride in Aromatic Hydrocarbons and Related Chemicals by Microcoulometry, ASTM International, West Conshohocken, PA, 2018 ( https://www.astm.org/Standards/D5805.htm)
- ASTM D7536 – 16, Standard Test Method for Chlorine in Aromatics by Monochromatic Wavelength Dispersive X-ray Fluorescence Spectrometry, ASTM International, West Conshohocken, PA, 2016 ( https://www.astm.org/Standards/D7536.htm)
Author: Joseph Iaia, Petroleum Product Manager

