- Analyzers
- Optics & Sources
- Technologies
- Support
- About
HDXRF vs ICP for Nickel and Vanadium in Crude Oil
BACKGROUND

Petra MAX ™ delivers advanced D4294 sulfur analysis in addition to 12 elements from P to Zn including Ni, V, and Fe. This robust benchtop analyzer complies with ASTM D4294 and ISO 8754 for measuring sulfur in hydrocarbons. Petra MAX is powered by HDXRF, utilizing XOS patented doubly curved crystal optics coupled with a high-performance silicon drift detector and an intense monochromatic excitation beam. This industry-leading technology reduces background noise and increases signal-to-noise output, enabling low detection limits and high precision without the need for consumable helium gas, a vacuum pump, or extensive sample preperation.
Technology introduced over the last decade, such as horizontal drilling and hydraulic fracturing, has led to new sources of light tight oil (LTO). LTO has grown in the US from essentially zero in 2010 to about 5 million barrels per day in 2017, exceeding the US production volume of non-tight oil. This trend is expected to continue with projections of 10 million barrels per day in the US by 2025, and significant supply in countries like Russia, China, Canada, Egypt, and Argentina. This is reshaping the landscape of available refining feedstock and challenges are arising across the industry. Refineries in the US Gulf Coast and across the world have invested significantly in processing units to handle much heavier crude oil. The new LTO contains significantly more naphtha than crude from conventional sources. Refiners are experiencing bottlenecks in the light ends distillation capacity and are having trouble keeping their conversion units, like the FCC, hydrocrackers, and cokers, full.
These changes are also having an impact on the quality of West Texas Intermediate (WTI) traded under the NYMEX Light Sweet Crude Oil (CL) futures contract delivered in Cushing, Oklahoma. The oil delivered is subject to specifications such as sulfur and API gravity, and oil blending near to the specification limit is common. Figure 1 plots the sulfur content of WTI delivered at Cushing. The sulfur content is consistently below the specified maximum of 0.42 wt% but never drops below 0.38 wt% as a result of oil blending. However, this blending creates new processing challenges for refiners as oils from other sources can introduce changing levels of other contaminants. Figure 2 plots the vanadium content of WTI delivered at Cushing, and depicts a trend toward higher levels over time. This is a result of blending oils from different sources with the WTI prior to delivery in Cushing. These changes in other oil quality parameters due to blending have led to many issues for refiners processing the crude oil. In response, NYMEX has amended rule 200101 to add five additional quality specifications including nickel and vanadium for contracts with delivery in January 2019 and beyond. The maximum concentrations allowed under the amended rule are 8 parts per million in nickel, and 15 parts per million in vanadium.
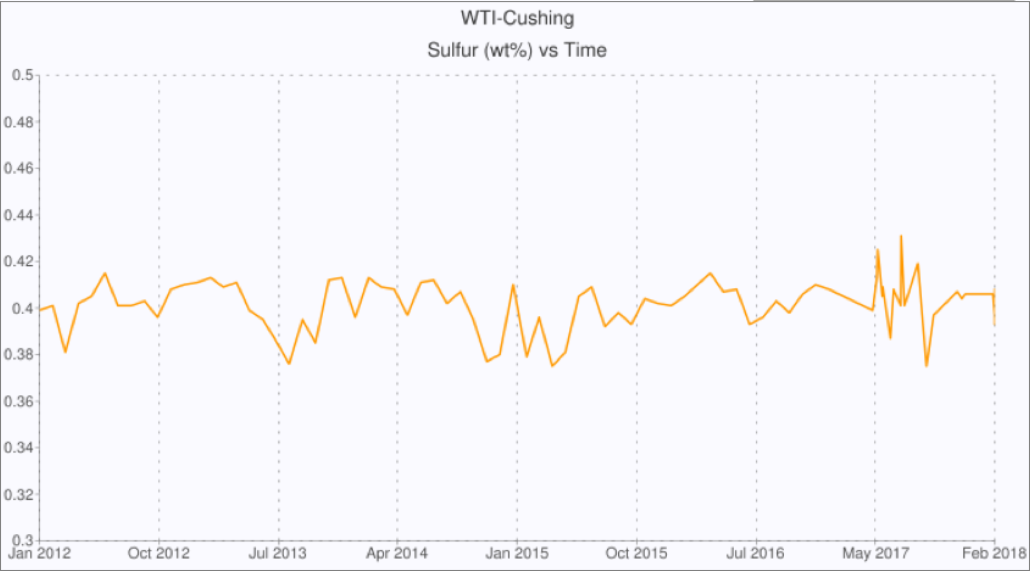
Figure 1: Sulfur Content of WTI Delivered at Cushing
Source: crudemonitor.us
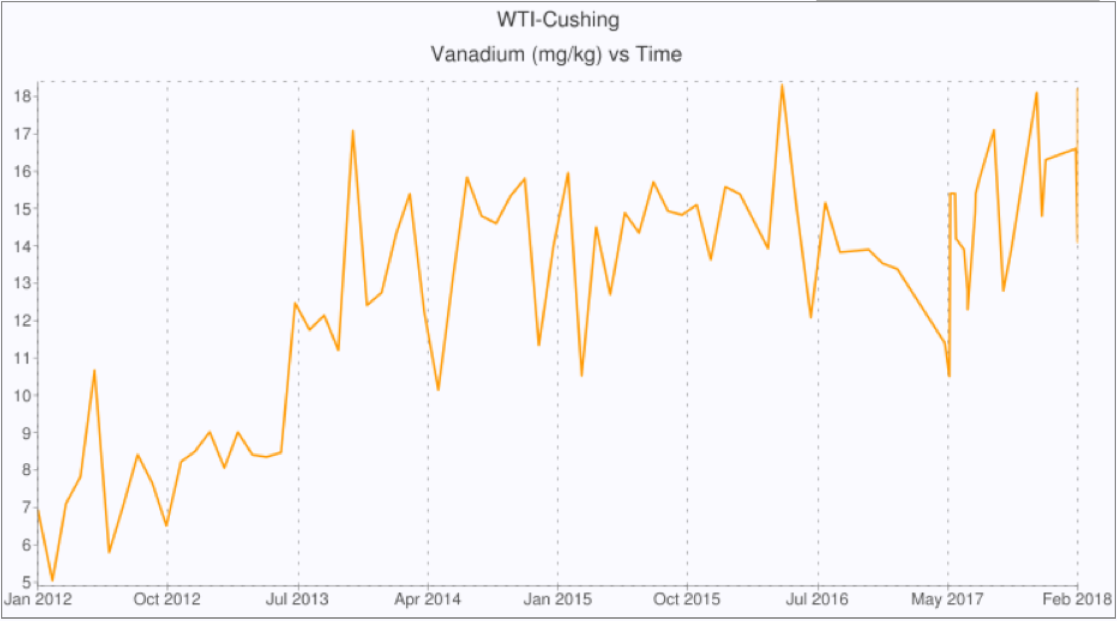
Figure 2: Vanadium Content of WTI Delivered at Cushing
Source: crudemonitor.us
CHALLENGE
Nickel and vanadium are naturally occurring in crude oil and become concentrated in the resids and heavier fractions of vacuum gas oils. They are known to rapidly deactivate cracking catalysts and can lead to off-specification coke, resulting in considerable costs to refiners. While refiners often look for opportunities to buy lower cost oils to improve profitability, understanding the content of contaminants like nickel and vanadium is important in order to adequately assess the impact on processing. Nickel and vanadium in crude oil can be tested using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) using ASTM test method D5708B. However, there are drawbacks to this technique. First, it requires a rigorous sample preparation process that involves strong acids, heating with hot plates, furnaces, and consumable gasses in a laboratory setting. Second, it is very time consuming: prep to analysis can take between 8 and 12 hours. Because of these drawbacks, ICP is not an efficient solution for analysis of nickel and vanadium in crude oil. In response, a faster, easier, and less expensive solution has been developed.
SOLUTION
X-ray Fluorescence Spectroscopy (XRF) is an alternative technology to ICP and most commonly used for sulfur analysis in liquid hydrocarbons like crude oil, fuels and lubricants. Utilizing standard methods like ASTM D4294 and ISO 8754, XRF is included in most crude oil specifications today. Petra MAX, a new XRF analyzer, delivers ASTM D4294 sulfur compliance with simultaneous measurement of nickel, vanadium, iron, and nine other elements at sub-ppm levels.
Petra MAX is powered by High Definition X-ray Fluorescence (HDXRF) technology: an elemental analysis technique offering significantly enhanced detection performance over traditional XRF technology. This technique applies state-of-the-art monochromating and focusing optics, enabling higher signal-to-background ratio compared to traditional polychromatic XRF. HDXRF does not require sample conversion, equating to no consumable gasses, little to no sample preparation, and delivers results in minutes.
Figure 3 shows the basic configuration of HDXRF and its use of focused monochromatic excitation. In this system, the diffraction-based doubly curved crystal optics capture a wide angle of X-rays from the source and focus a narrow energy band (monochromatic) of X-rays to a small spot on a measurement cell. The monochromatic beam excites the sample and secondary characteristic fluorescence X-rays are emitted. A detector processes the secondary X-rays and the instrument reports elemental composition of the sample.
Figure 3: HDXRF Technology
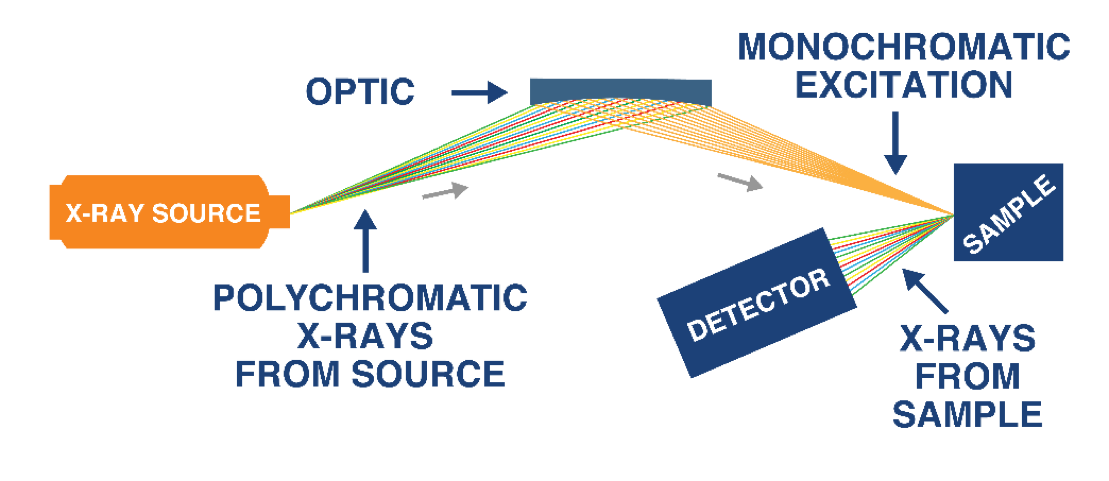
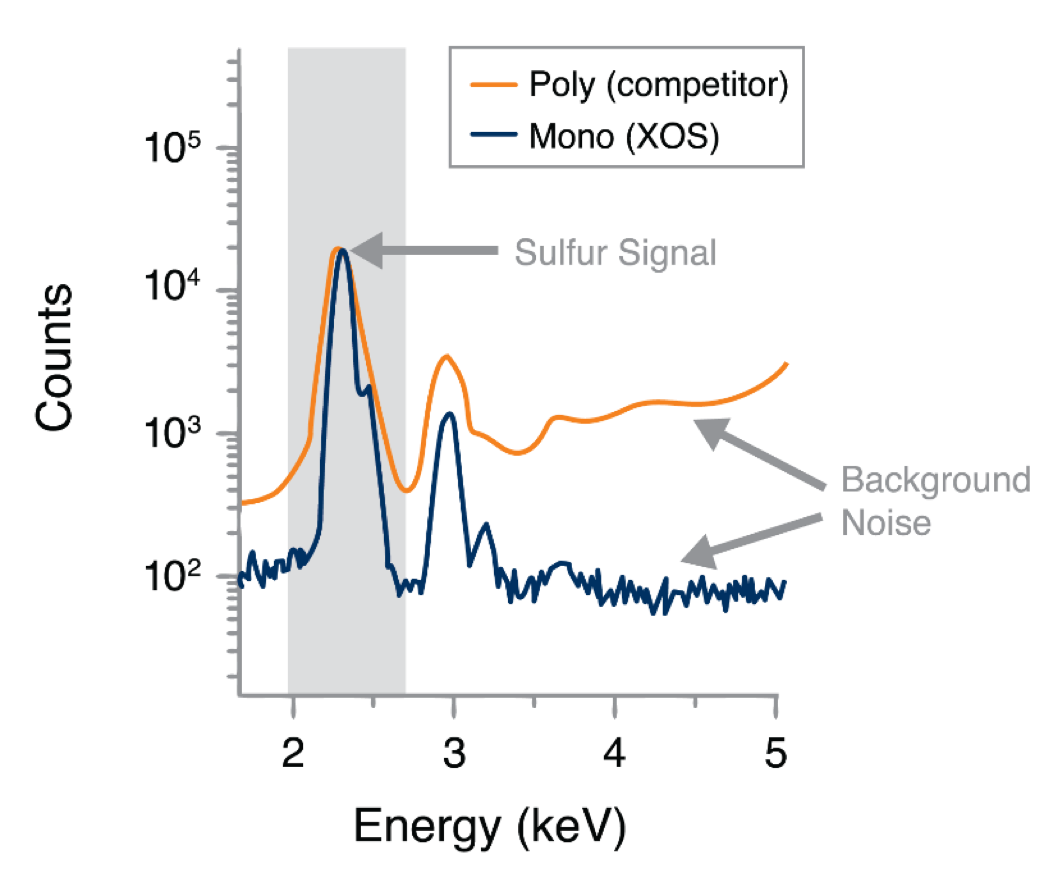
Figure 4 compares the detector signal of polychromatic (competitor) with monochromatic (XOS) XRF to demonstrate how monochromatic excitation reduces background noise and improves signal definition, delivering lower limits of detection and dramatically better precision.
Figure 4: Superior Signal-to-Noise Ratio
HDXRF VS ICP STUDY
A study was conducted to compare the sample preparation process and precision using Petra MAX, powered by HDXRF, and ICP to measure nickel and vanadium in crude oil. Four crude oil samples were obtained for the comparison study:
A. Custom doped crude oil standard from VHG LabsB. Sour crude oil retain from Intertek
C. Medium sour crude retain from Crudemonitor.ca
D. Heavy sour crude retain from Crudemonitor.ca
Three independent laboratories analyzed the sample set using ASTM D5708B (ICP) and Petra MAX (HDXRF). Each participant received two randomized sample sets packaged in blind duplicate for analysis. The resulting raw data sample means can be seen in Table 1.
| Table 1: Raw Data Sample Means | ||||
|---|---|---|---|---|
| Sample | ICP – D5708B | Petra MAX (HDXRF) | ||
| Ni (ppm) | V (ppm) | Ni (ppm) | V (ppm) | |
| A | 7.4 | 14.2 | 8.7 | 14.3 |
| B | 5.2 | 17.9 | 5.6 | 16.7 |
| C | 12.4 | 31.5 | 12.7 | 29.9 |
| D | 30.5 | 73.9 | 45.3 | 100.4 |
ICP Sample Preparation Notes
ICP performed by ASTM method D5708B requires extensive sample preparation when analyzing crude oil. Crude oil is first transferred into a glass beaker and weighed. The sample is digested with sulfuric acid and heat, using both an infrared lamp and a hotplate. The sample is stirred continuously with a glass rod during digestion and carefully monitored to ensure that no sample is lost due to frothing and spattering. Once the foaming has finished, the heat is increased until the sample has been reduced to a carbonaceous ash. This process is completed inside a wellventilated fume hood using gloves and a face shield for protection from strong oxidizing fumes generated during digestion. This ash is then carefully transferred to a muffle furnace and all of the carbon is burned away. The remaining material is then reconstituted with nitric acid and returned to a steam bath. This beaker is then transferred to a hotplate where it remains until the liquid has fully evaporated. Once again, nitric acid is added and the sample is transferred to a volumetric flask and brought to a known volume with additional nitric acid. The sample is then ready for analysis. Users report that this process of sample preparation to results takes anywhere from 8 to 12 hours to complete.
HDXRF Sample Preparation Notes
Samples prepared for analysis by Petra MAX are first shaken to homogenize the sample. Using a disposable pipette, 7 to 10 mL of sample is transferred to a disposable plastic sample cup designed for XRF analysis. An X-ray transparent film is then placed over the opening of the cup and affixed using a snap-on ring. Finally, the sample is placed in the instrument and ready for analysis. This process from sample preparation to results takes about 6 minutes.
HDXRF VS ICP CORRELATION
A simple way to show correlation between techniques is to measure a sample set spanning a range using two different techniques. Using a spreadsheet, scatter plot the results of the study with each technique on a separate axis. Next, plot the trend line with the R-squared value, also known as the coefficient of determination. This is a value between 0 and 1. The better the correlation, the closer this value will be to 1. If the correlation between the two techniques is good, the plotted data points will be on or near the trend line, and the R-squared value will be close to 1. If the correlation is poor, the data points will not be near the trend line, and the R-squared value will be much less than 1. Figure 5 depicts an example of good correlation, and Figure 6 depicts an example of poor correlation. Figure 7 shows the correlation between Petra MAX and ICP as a result of this study. The plotted points are near the trend lines for both nickel and vanadium, and the R-squared value for both elements is 0.99. This indicates that there is good correlation between Petra MAX and ICP for nickel and vanadium in crude oil.
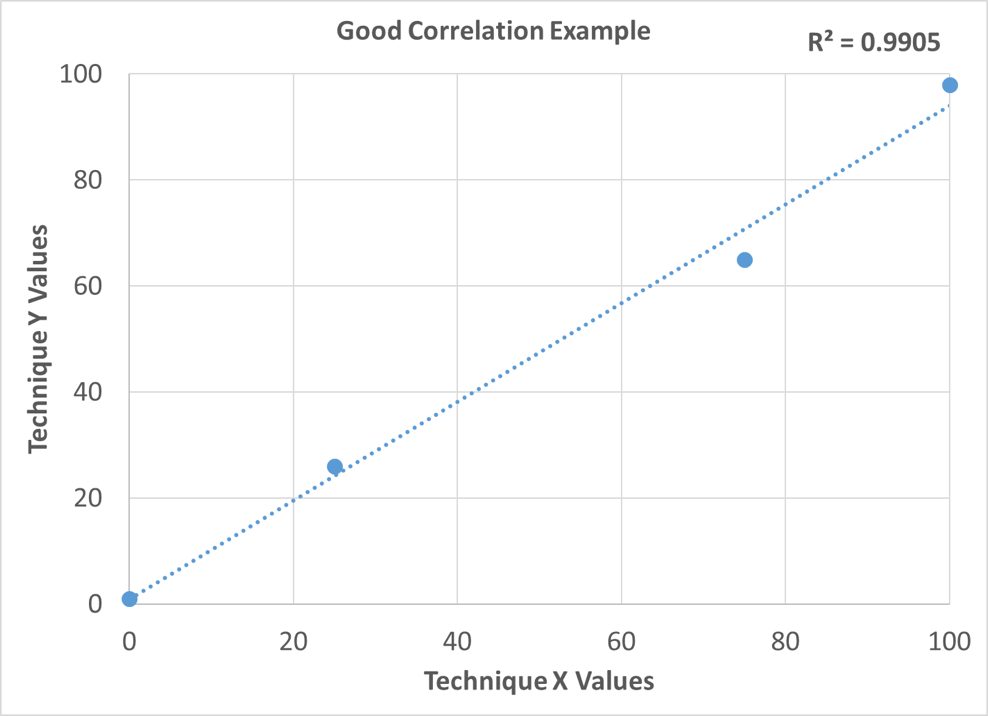
Figure 5: Good Correlation
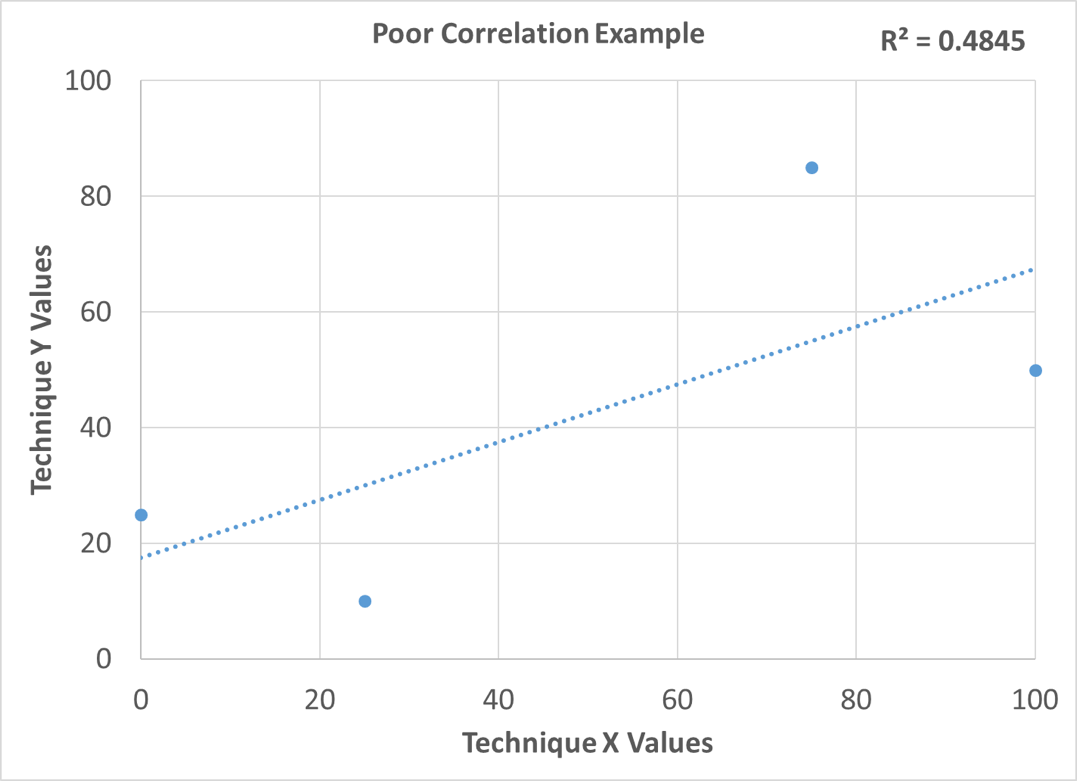
Figure 6: Poor Correlation
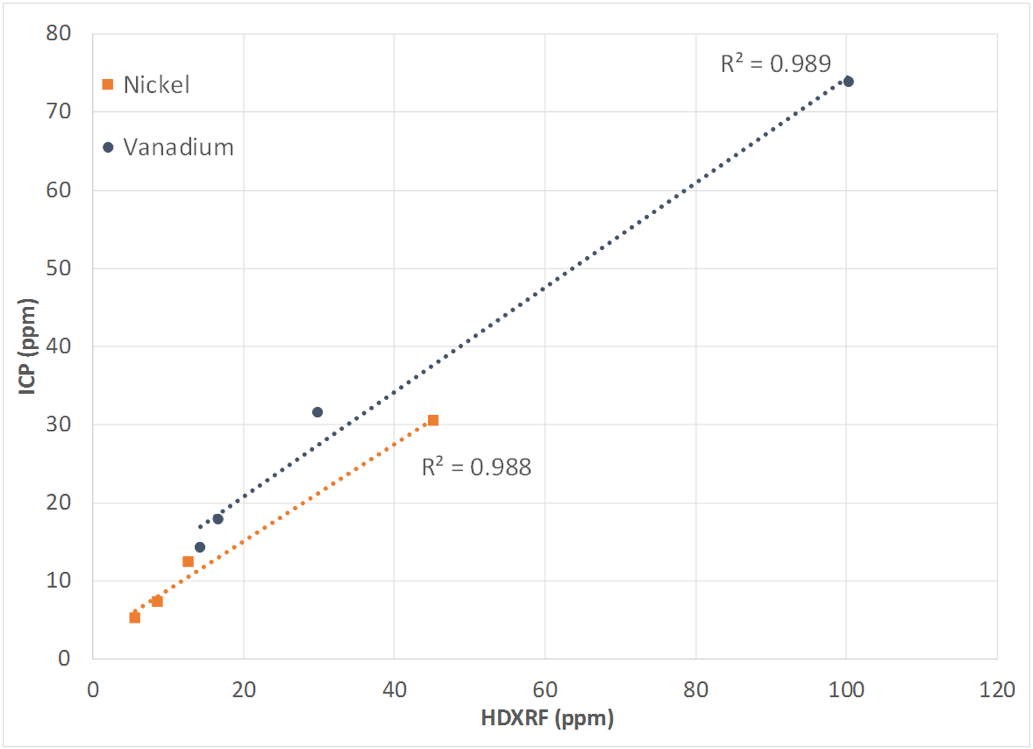
Figure 7: ICP vs Petra MAX Correlation
PETRA MAX VS ICP PRECISION
Precision is an important characteristic of measurement technologies. Because a single measurement is typically used to represent an important quality parameter like sulfur, nickel, or vanadium, it is important to understand how much variability is associated with the measurement value. The more precise a measurement technique is, the less likely that undesirable results will occur. In the case of crude oil, a buyer and seller are less likely to dispute whether quality specifications have been satisfied, and the refiner can be sure they are taking the proper considerations during processing. ASTM and ISO standard test methods evaluate the precision of a test method in terms of repeatability and reproducibility.
Repeatability (r) is defined as the difference between repetitive results obtained by the same operator in a given laboratory, applying the same test method with the same apparatus, under constant operating conditions, on identical test material and within short intervals of time, would in the long run and in the normal and correct operation of the test method, exceed the value calculated only once in 20 measurements (5% of the time). Or more simply put, repeatability is the maximum expected difference (at 95% confidence) between two measurement results run on the same material using the same apparatus, test method, and operator.
Reproducibility (R) is the difference between two single independent results obtained by different operators, applying the same test method in different laboratories, using different apparatus on identical test material, would in the long run and in the normal and correct operation of the test method, exceed the value calculated only once in 20 measurements (5% of the time). Or, reproducibility is the maximum expected difference (at 95% confidence) between two measurements taken on the same material using the same test method by two different laboratories each using a different apparatus and operator.
Precision is often dependent on the concentration level of the material being tested, and in these cases will be expressed as equation. Precision equations for elemental analysis test methods are generally linear or exponential as in the following examples:
- Linear precision example:
(r) or (R) = X * 0.1234 - Exponential precision example:
(r) or (R) = X0.123 * 4.567
In these precision examples, X is the mean value or concentration of interest. To improve visualization, precision statements are often graphed with the concentration (X) on the x-axis and the corresponding repeatability or reproducibility on the y-axis. The lower the value on the y-axis for a given concentration, the better the precision. Figure 8 depicts an example of good precision, and Figure 9 depicts an example of poor precision (as compared to the good precision plot).
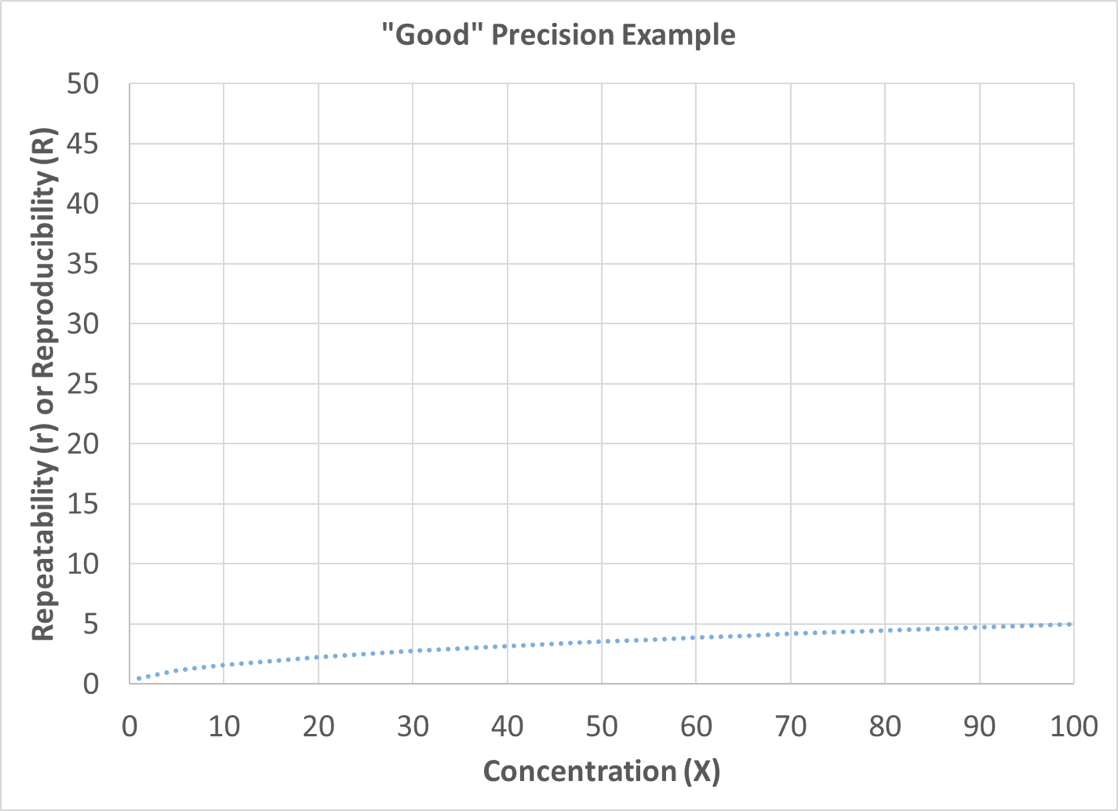
Figure 8: Good Precision
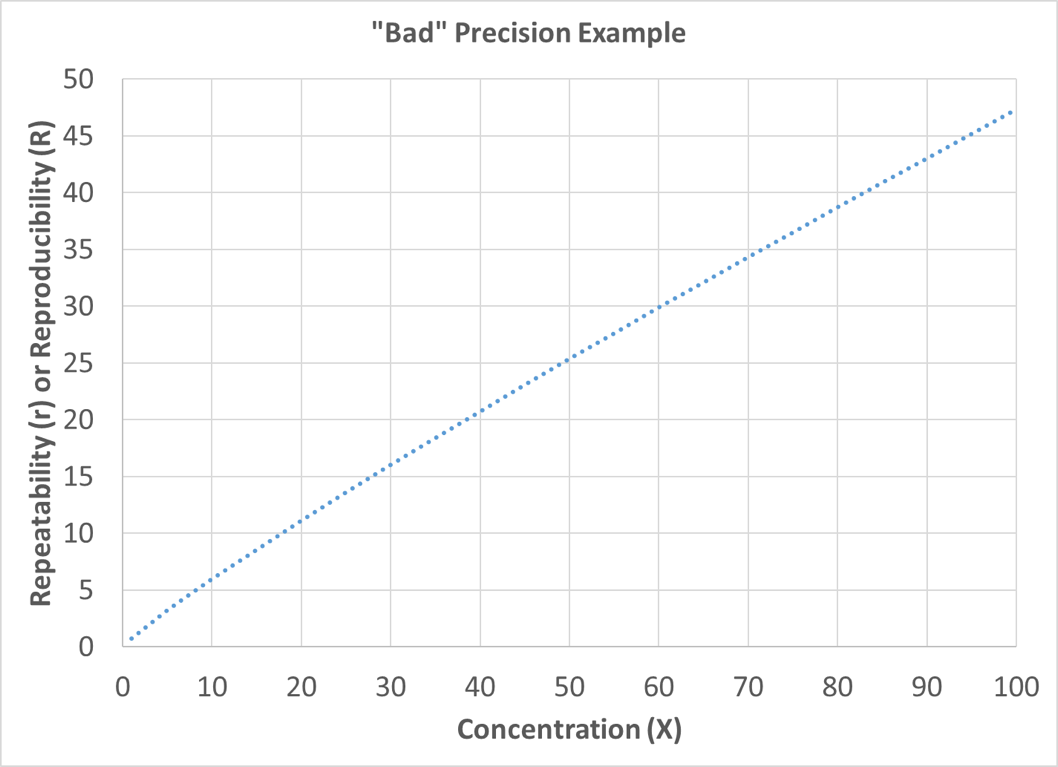
Figure 9: Poor Precision
Precision of the study data was calculated in accordance with ASTM D6300, Standard Practice for Determination of Precision and Bias Data for Use in Test Methods for Petroleum Products and Lubricants, and is depicted in graphical format in Figure 10-13, with repeatability in Figure 10 and 11, and reproducibility in Figure 12 and 13. The precision of the study data is depicted in solid blue (HDXRF) or orange (ICP) lines. For context, the precision of ASTM test method D5708B (ICP after acid decomposition) has been added as a dotted orange line to all figures.
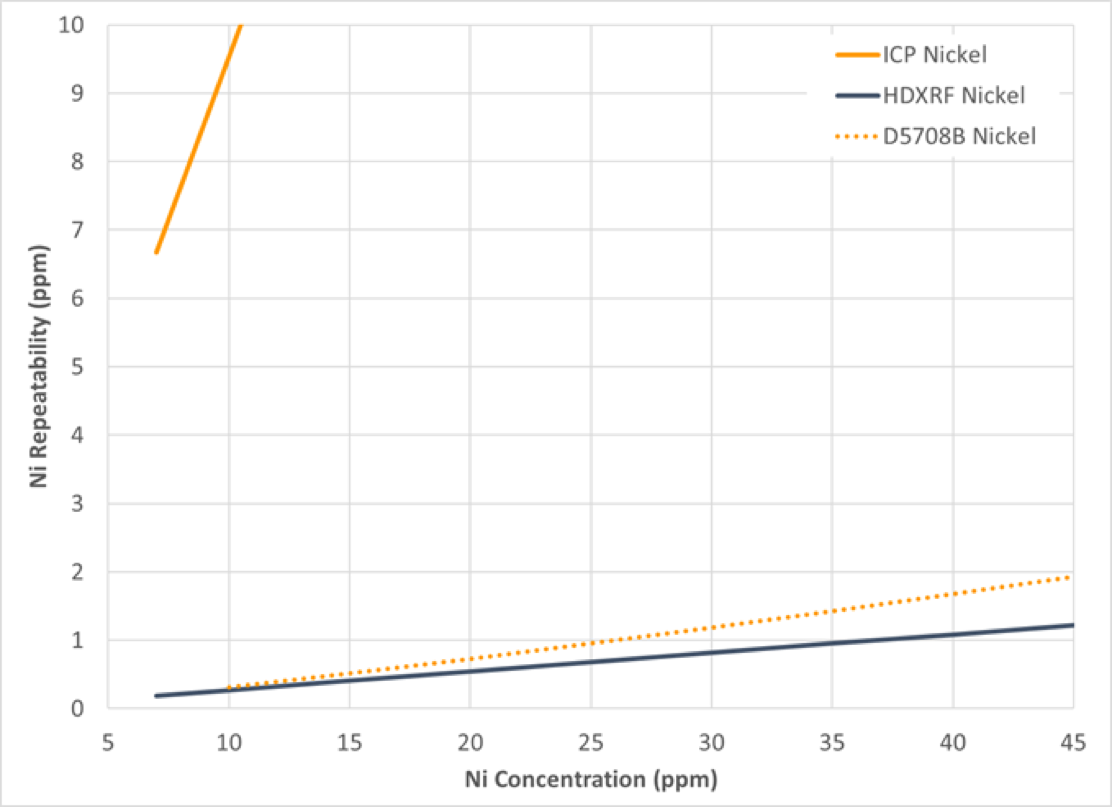
Figure 10: Nickel Repeatability
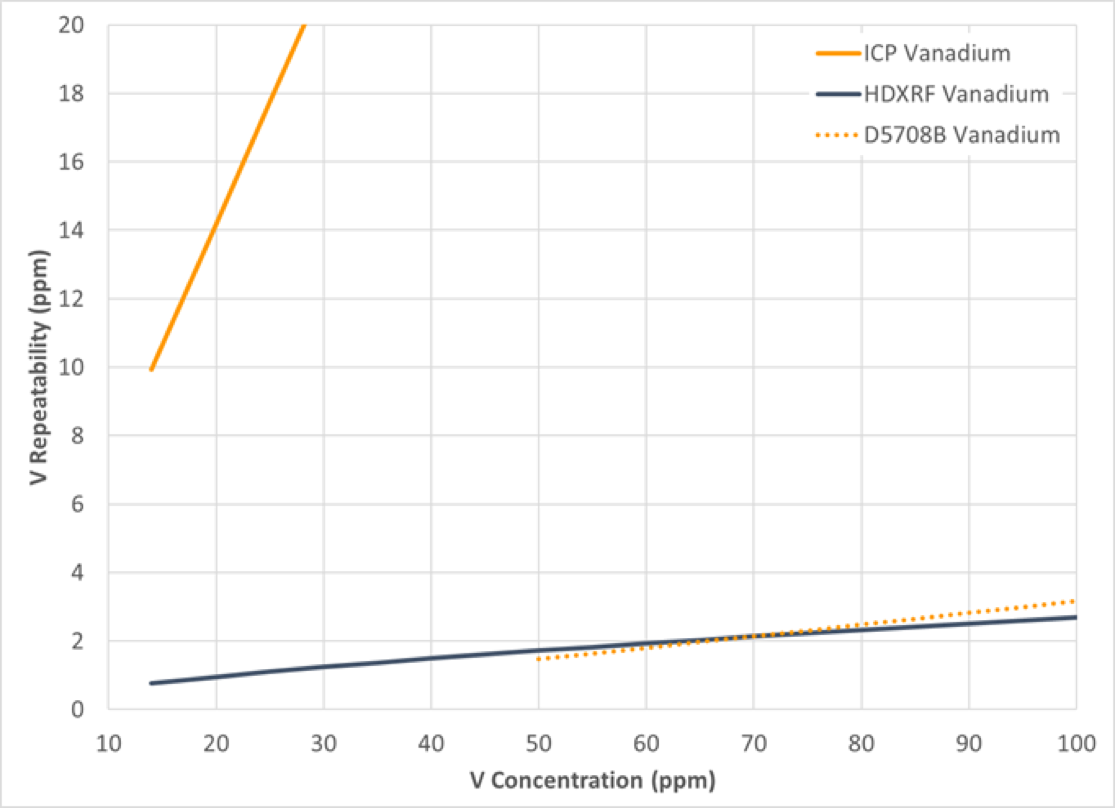
Figure 11: Vanadium Repeatability
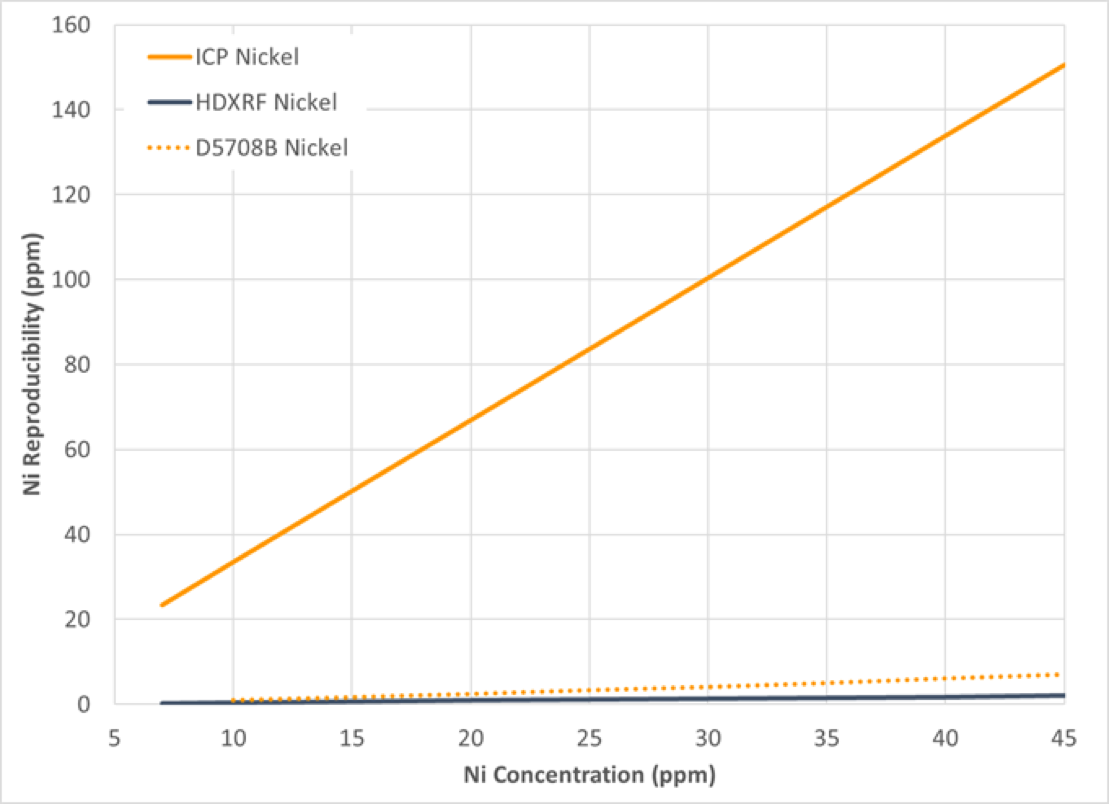
Figure 12: Nickel Reproducibility
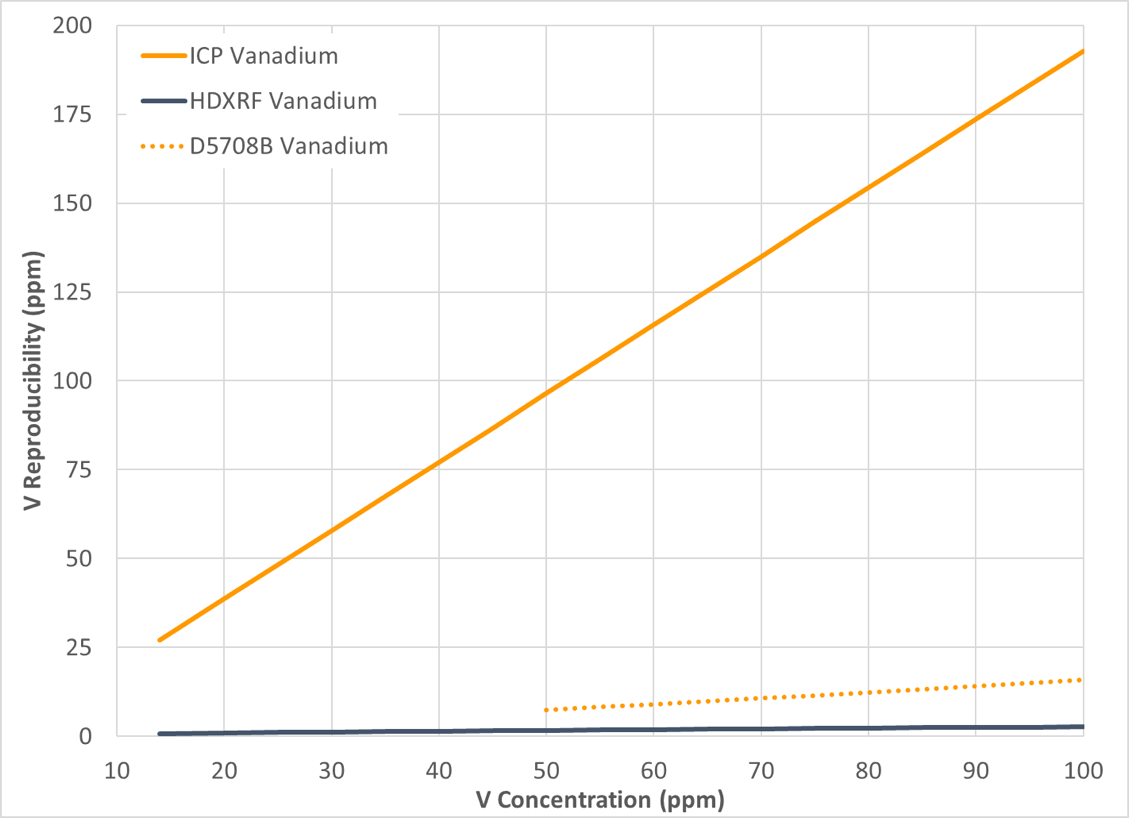
Figure 13: Vanadium Reproducibility
The results of this study show that Petra MAX, powered by HDXRF, is more efficient and delivers precision comparable or better than the ICP ASTM test method precision (D5708B) for nickel and vanadium in crude oil.
For both nickel and vanadium, the ICP repeatability and reproducibility results demonstrated poorer precision than expected, as shown in Figures 10, 11, 12, and 13. To understand why, there are a few possible explanations to consider:
ASTM D5708B method scope covers both crude oils and residual fuels. When the ASTM D5708B precision study was conducted, samples were included that covered not only crude oils, but also residual fuels. In our study, only crude oils were included. It may be that residual fuels are easier to prepare and analyze than crude oil by ASTM D5708B. The additional data from residual oil to the overall precision statement of ASTM D5708B may lower the overall precision from what you would see when only crude oil samples are included.
Sample D was a highly bituminous crude oil from Canada. This type of sample represents one of the most challenging sample types to measure by ASTM D5708B, because bituminous samples are difficult to digest. This leads to measurement variability, and poorer precision for this sample type. This is further illustrated in the next section. Conversely, the precision of the Petra MAX is less affected by this sample type due to the minimal sample preparation needed for this analyzer.
BOX AND WHISKER PLOT COMPARISON
Another way to compare both correlation and precision is by creating a box and whisker plot. This type of plot shows a quick graphical examination of sample results distribution. See Figure 14 for the anatomy of a box plot. The box encompasses the 25th through 75th percentiles, with a line drawn at the median. The larger the box the wider the sample distribution, therefore, a smaller box indicates tighter precision. If the 50% median bisects the center of the box, this indicates a normal distribution (bell curve). If it is closer to one end or the other, then the distribution is skewed. When comparing two methods, the relative size of the box is indicative of precision, and the median is used to infer correlation. In the example in Figure 14, method B has better precision than method A, and the methods show good correlation because their medians are similar.
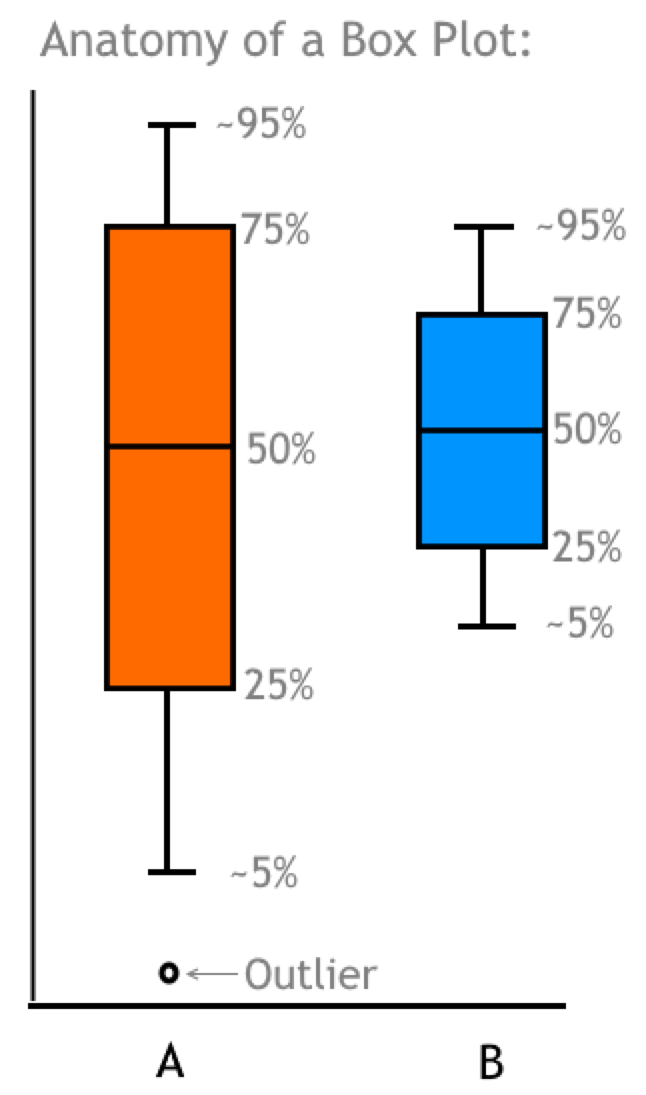
Figure 14: Box Plot Example
Figures 15 and 16 are box and whisker plots of the study data for nickel and vanadium by HDXRF and ICP, broken out by sample. ICP sample distribution is shown in orange, and HDXRF sample distribution is shown in blue. Additionally, the gravimetrically doped concentration of sample A is plotted as a red dot to illustrate the expected value. The medians for both Petra MAX and ICP are very close to the gravimetric value, and are similar for crude oil samples B, C, and D, indicating good correlation between techniques. However, the variability of individual ICP results is clearly much higher than Petra MAX, indicating that Petra MAX has better precision than ICP. The box plots also demonstrate how bituminous crude oil (sample D) has a negative impact on precision, especially for ICP.
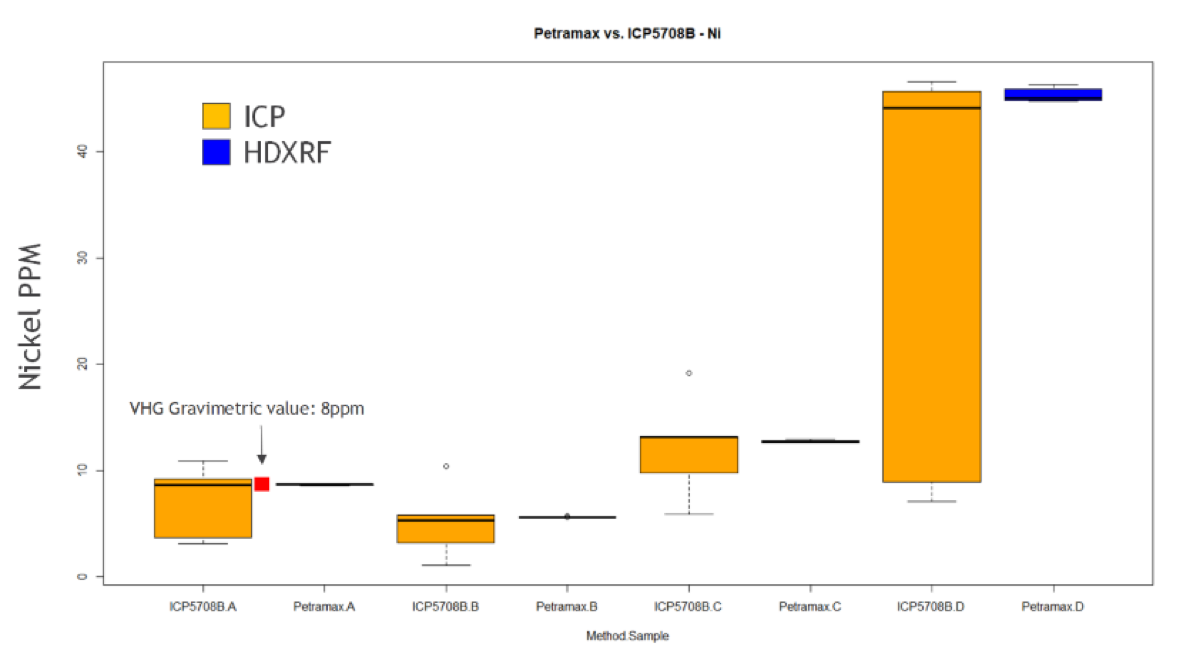
Figure 15: Nickel Box Plot
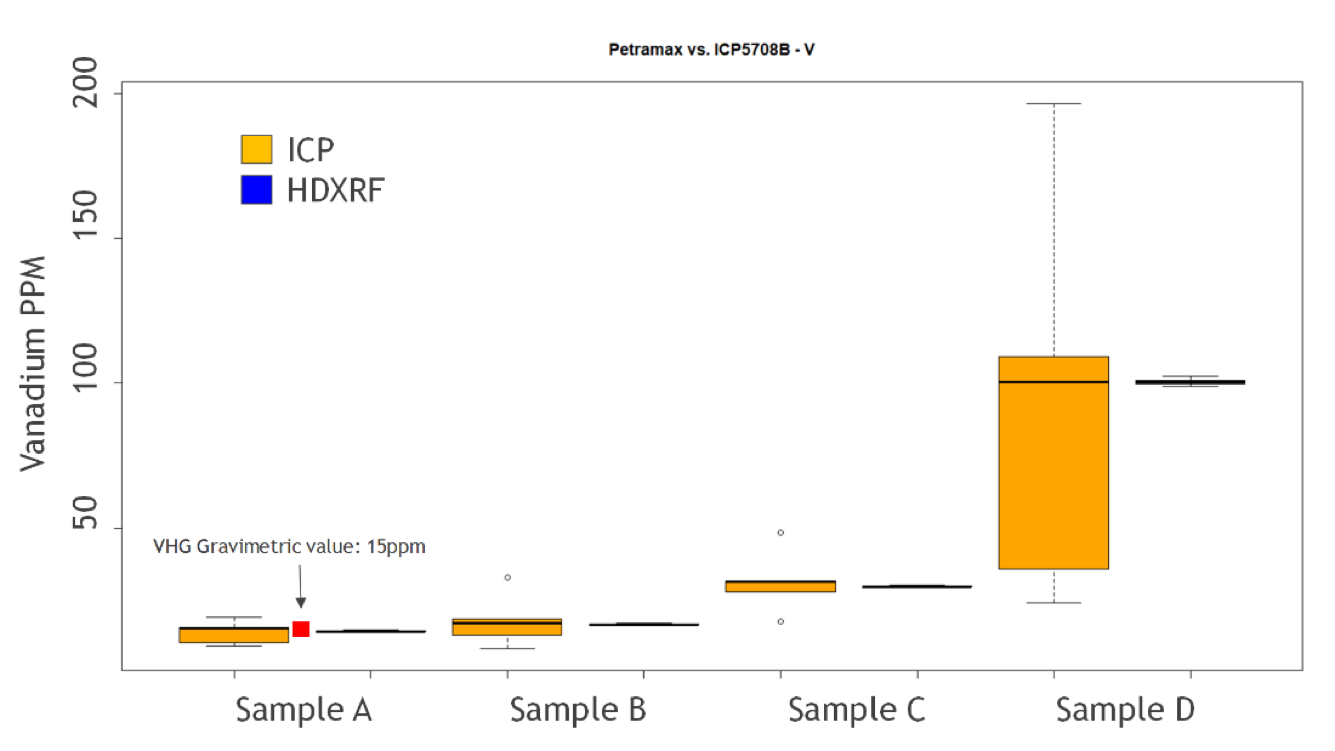
Figure 16: Vanadium Box Plot
Conclusion
Sulfur has long been a critical quality parameter for crude oil. Changes in crude oil production technologies have resulted in an increased importance of monitoring nickel and vanadium. This study demonstrates that HDXRF shows good correlation with ICP for nickel and vanadium in crude oil. Petra MAX, powered by HDXRF, shows better precision and lower variability when compared to ICP for the crude oils studied. With minimal sample preparation and rapid results, Petra MAX is an ideal solution for petroleum laboratories.
Authors: Kyle Kuwitzky, Senior Product Manager and Leslie Johnson, Applications Scientist

