- Analyzers
- Optics & Sources
- Technologies
- Support
- About
Monitoring Chlorine to Prevent Refinery Corrosion
Print

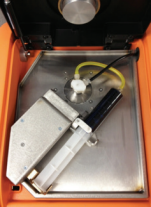 Figure 1 - Clora Accu-Flow Design
The Accu-Flow design works for crude oils with a maximum viscosity of 2000 cSt at 70°F. A 60 ml crude oil sample is injected at a 20 ml/ min flow rate into the yellow tubing (Figure 1). The sample flows from the yellow tubing through the Accu-Flow insert assembly, where the sample is exposed to X-rays. The sample then flows through the black drain tube where it is collected outside the analyzer. Typical measurement time is three minutes. Depending on the crude viscosity and rate of inorganic chloride settling, the difference between static and Accu-Flow measurement results can be quite dramatic. In the following examples, four samples of two crude oils have been run either statically or using Accu-Flow for a three minute measurement time. In order to illustrate the behavior of chlorine during the measurement, 30 second data points have been taken within the three minute measurement interval and graphed accordingly. In each case, the static measurement increases over time (Figures 2 and 3), leading to an inaccurate total chlorine measurement. However, the AccuFlow measurement remains stable throughout the measurement period (Figures 2 and 3) and is repeatable over multiple measurements as seen in Table 1.
Figure 1 - Clora Accu-Flow Design
The Accu-Flow design works for crude oils with a maximum viscosity of 2000 cSt at 70°F. A 60 ml crude oil sample is injected at a 20 ml/ min flow rate into the yellow tubing (Figure 1). The sample flows from the yellow tubing through the Accu-Flow insert assembly, where the sample is exposed to X-rays. The sample then flows through the black drain tube where it is collected outside the analyzer. Typical measurement time is three minutes. Depending on the crude viscosity and rate of inorganic chloride settling, the difference between static and Accu-Flow measurement results can be quite dramatic. In the following examples, four samples of two crude oils have been run either statically or using Accu-Flow for a three minute measurement time. In order to illustrate the behavior of chlorine during the measurement, 30 second data points have been taken within the three minute measurement interval and graphed accordingly. In each case, the static measurement increases over time (Figures 2 and 3), leading to an inaccurate total chlorine measurement. However, the AccuFlow measurement remains stable throughout the measurement period (Figures 2 and 3) and is repeatable over multiple measurements as seen in Table 1.
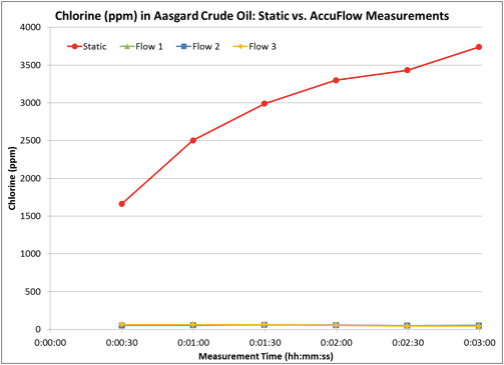
Figure 2 - Chlorine in Aagard Crude Oil: Static vs. Accu-Flow
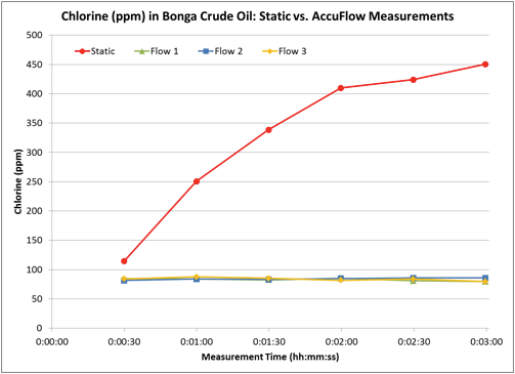
Figure 3 – Chlorine in Bonga Crude Oil: Static vs. Accu-Flow
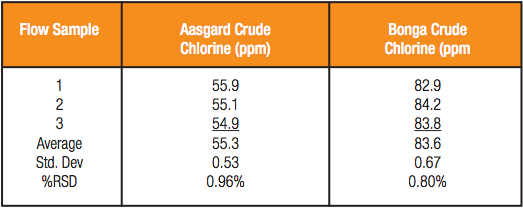
Table 1 – Average Chlorine Concentration of Accu-Flow Repeats
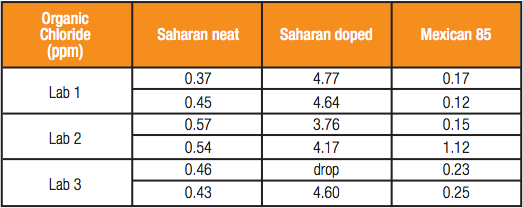
Table 2 – Organic Chloride Content by ASTM D4929 Distillation and MWDXRF Analysis
For more information on chlorine analysis using MWDXRF technology, please visit https://www.xos.com/products/chlorine-analyzers

Chlorine in crude oil, if not removed, can hydrolyze during processing to form hydrochloric acid. The crude oil desalter is the first line of defense in the prevention of corrosion. However, in order to provide a proper defense, the correct chloride monitoring must be put in place.
Unfortunately, many refiners rely on semi-periodic testing of inorganic chlorides to get the job done. However, what if a desalter upset occurs in between testing periods? Worse yet, what if an organic chlorine slug is present in the incoming crude? The desalter will remove only inorganic chlorides, not organic chlorides, and any chlorides that pass through the desalter have the potential of causing fouling and corrosion issues.
Even refiners who don’t rely on semi-periodic testing typically only monitor inorganic chlorine. While this is very important for typicallyoccurring inorganic salts and for monitoring desalter efficiency, this does not capture all the threats; this is why refiners should also be looking at total chlorine.
X-ray Fluorescence (XRF) Spectrometry, while widely used for total sulfur testing, has traditionally been an ineffective tool for the direct measurement of total chlorides in crude oil, due to the settling of inorganic chloride salts during the measurement process. This settling leads to poor precision and an affinity for other measurement or sample extraction techniques.
Recently XOS introduced a continuous flow option to their Monochromatic Wavelength Dispersive XRF (MWDXRF) benchtop chlorine spectrometer, called the Clora Accu-Flow. The Clora Accu-Flow uses a stepper motor for continuous injection of crude oil during the sample measurement, eliminating the settling of inorganic chloride salts and allowing for the direct measurement of total chlorine without water wash extraction or sample distillation.


Figure 2 - Chlorine in Aagard Crude Oil: Static vs. Accu-Flow
Apllication Note

Figure 3 – Chlorine in Bonga Crude Oil: Static vs. Accu-Flow

Table 1 – Average Chlorine Concentration of Accu-Flow Repeats
For those interested in a continuous chlorine monitoring system, XOS’s Clora analyzer is also available in an on-line version: the Clora On-Line Analyzer. This on-line analyzer can accept most crude oil streams with a maximum viscosity limitation of 160 cSt. More viscous materials can be analyzed by increasing sample temperature up to 275°F. Though the typical complete measurement time is five minutes, the analyzer also provides a 15-second snapshot analysis.
Although all XRF techniques are capable of only total elemental analysis, with some sample preparation, the benchtop Clora and Clora Accu-Flow can also be used to characterize inorganic and organic chlorine in crude oil. Using a hot water extraction, crude oil may be separated into its organic chloride and inorganic chloride constituents with the organic chlorides staying in the crude oil layer and the inorganic chlorides precipitating into the water layer. The Clora can then be used to measure each layer to determine organic and inorganic chlorides. While many laboratories have successfully used this sample preparation technique, unfortunately, this technique has crude-dependent limitations. Not all crude oils can be easily extracted and this sample preparation technique has variable within-lab repeatability and poor between-lab reproducibility.
Use of the Clora for testing organic chlorides in crude oil via a modified ASTM D4929 method shows more promise for better repeatability and reproducibility. ASTM D4929, Standard Test Methods for Determination of Organic Chloride Content in Crude Oil, is an industry-wide standard using distillation of crude oil to the naphtha cut and subsequent analysis of organic chlorides by sodium biphenyl reduction and potentiometry, or combustion and microcoulometry.
Data presented at ASTM indicates that MWDXRF is a promising alternative to potentiometry or microcoulometry for the analysis of organic chlorides in crude oil. Three independent third-party test laboratories were given three crude oil samples in blind duplicate. Two samples were raw crude oils, Saharan and Mexican 85. The third sample was the Saharan crude oil doped nominally with 4.3 ppm organic chlorine. The laboratories were instructed to analyze the crude oils by D4929 distillation and subsequent chlorine analysis by MWDXRF.
The results in Table 2 were favorably received by ASTM D02.03 subcommittee on Elemental Analysis within the D02 Petroleum Products committee. Permission was given by the subcommittee to run a formal Interlaboratory Study for the incorporation of XRF into ASTM D4929 as test method C for determination of organic chloride content in crude oil. The Interlaboratory Study is currently in progress and results are expected to be presented to ASTM D02.03 at their December 2015 meeting.

Table 2 – Organic Chloride Content by ASTM D4929 Distillation and MWDXRF Analysis
For more information on chlorine analysis using MWDXRF technology, please visit https://www.xos.com/products/chlorine-analyzers

