- Analyzers
- Optics & Sources
- Technologies
- Support
- About
Single Crystal Diffraction and Protein Crystallography
Since protein and other biomacromolecular crystals are very weak diffractors, it is often desirable to obtain increased flux on the crystal with conventional laboratory X-ray sources. Single crystal diffraction or protein crystallography measurements can be enhanced by using either polycapillary collimating or polycapillary focusing optics. Contrary to traditional protein crystallography, convergent beam systems using capillary optics can be used for screening very small protein crystals, and micro diffraction measurements can be made using a very low-powered source. Polycapillary optics can be used in conjunction with low-power X-ray sources to achieve X-ray densities in small beam spots, resulting in diffracted-beam intensities equal or greater than that achieved using rotating anode sources equipped with the most advanced confocal optics. The unit cell size that can be successfully analyzed with collimating or slightly focusing optics is < 200?÷. Even smaller unit cell sizes can be measured with micro X-ray diffraction using focusing optics for more strongly convergent beams.
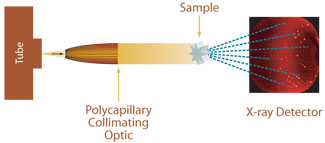
Single crystal parallel beam X-ray diffraction using a polycapillary collimating optic
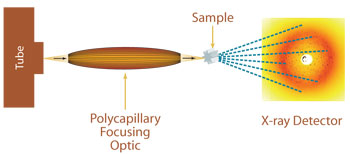
Single crystal convergent beam X-ray diffraction using a polycapillary focusing optic
The diffracted-beam intensity obtained with a polycapillary monolithic optic with a slightly convergent beam and a 50 W microfocus source was equal to or greater than that from a 5 kW rotating anode source equipped with the most advanced confocal optics. Contrary to traditional protein crystallography, convergent beam systems using capillary optics can be used for screening very small protein crystals, and micro diffraction measurements can be made using a very low-powered source.

